With a good rechargeable battery attached to your solar PV system will ensure the efficiency of your power generation and storage is optimized as all the power generated from solar PV panel are only when there is sunlight and you will need to depends on battery storage when there is no sunlight.
However before buying any battery and attached to a charge controller, it is important to make a right choice at the beginning, or else will run into issues such as early system failure due to incorrect matching of devices, budget busting high initial cost on unnecessary items, or high maintenance due to frequent battery replacement. A good battery will ensure your system can deliver the power when you need most and able to last.
Important terminology
Before we start explaining battery types, lets see some important terminology in regard to rechargeable battery system.
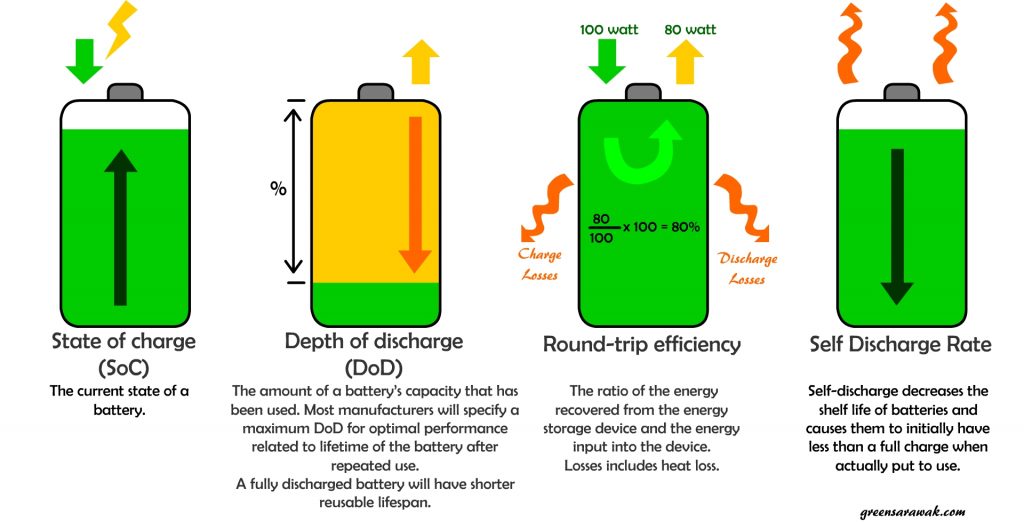
State of Charge
State of charge of a battery is easily understand as how much a battery is charged during a charge cycle. Similarly to a fuel tank meter that tells you how full is your fuel tank in a automobile.
There is many ways to tell how full is a battery. Common method includes chemical testing (e.g using hydrometers to calculate the specific gravity of a battery water), Voltage testing (e.g. Using Voltmeter and compare with a look-up table of the battery’s open circuit voltage vs temperature) and Current integration method (e.g measuring battery current and integrating in time).
It is easily seen that, if the state of charge of a battery drops, the other parameters also follows. However the voltage (and some time combined with current calculations) is deem the easiest and less messy method.
For example, a 12 Volt flooded lead acid battery will have specific gravity (SG) of 1.277 and a open circuit voltage (Voc) of 12.73V when the battery is at fully charged state. At 50% state of charge (SoC), the SG will drop to 1.172 and Voc will drop to 12.10V. At 10% SoC, the SG will drop to 1.073 and Voc will drop to 11.51V.
It is important that the accurate measurement of State of Charge is depends on battery type and technology. For easy comparison, go back to the manufacturer datasheet to understand the state of charge of your battery.
Depth of Discharge
Depth of discharge (DoD) is opposite of state of charge of a battery. A fully charge battery with 100% SoC will have DoD of 0%. One number goes up and the other comes down and vice versa. So why the two terms ?
Basically DoD is use in correlation to the cycle life of a battery. The general rule of thumb is, the more we frequently discharge a battery, the shorter the life span of the battery, due to chemical changes in the battery and internal resistance build up. Hence a shallow discharge cycle will allow the life span of the battery to be longer.
In off-grid solar system we take DoD quite seriously as we had a limited time of a day with sun light to charge the battery while the other time we will be draining the power solely from the battery. Improper planing will cause the battery to damage faster and increase the maintenance cost.
A good quality deep cycle battery can have lifespan of nearly 10 years if it was handled properly, but it can also spoil within half years of usage if you abuse it everyday.
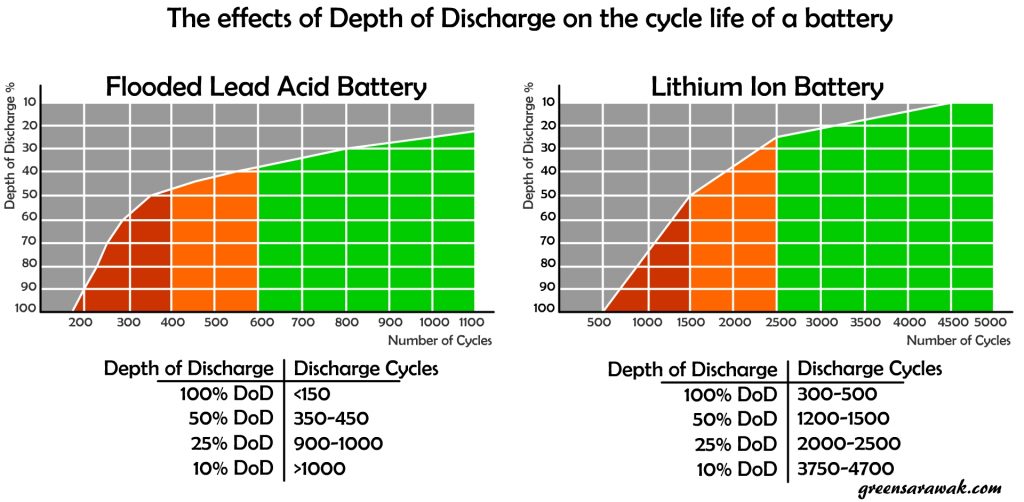
Different type of battery have different rated cycle life and recommended Depth of Discharge. Lithium ion battery is generally rated as one of the good candidate for long lasting battery with more cycle life usage, as long as not abuse with extensive discharge.
It is important to look at the manufacture datasheet on the recommended Depth of Discharge and its rated cycle life. Some battery like Lithium Ion Battery will usually show the cycle life of the battery with DoD of 80%, while flood lead acid battery will usually show the cycle life of the battery with DoD of 50% or 25%. Reason been, since the solar battery are charged once a day when the sun was out, each charge considered one cycle and 1000 cycles will ensure you can use it without issues within 2.5-3years if used with the stated DoD recommendation. Most batteries don’t come with long warranties.
With DoD in mind, it allows you to carefully plan for your battery system. Remember your load calculation ? if you required a daily dose of 100 amp-hours of energy, to ensure it is within 50% DoD, you need 200 amp-hours of battery capacity. If you want the battery system to last even longer and target for a 25% DoD, you need a 400 amp-hours of battery capacity.
Some battery like the lithium ion battery will have a battery management circuit (BMS) that will shut down the cells to prevent further discharge that will damage the battery if it gets below a certain voltage.
Round-trip efficiency
Round-trip efficiency is usually express in percentage. It is the ratio of the energy that is put into the storage to the usable energy that can be retrieve from the storage.
A high round-trip efficiency storage will means there is less energy been lost from the storage.The general round-trip efficiency of a battery falls between 75% – 90% depends on battery type.
These loss of energy is mainly due to heat dissipation during charging and discharging. Some of the energy loss in the form of chemical reaction and internal resistant.
Self Discharge Rate
Self-discharge is a normal phenomenon of a chemical battery. Due to internal chemical reaction in a battery, it can slowly loss its charges over time even when it is not connected or used. Rechargeable battery have higher self discharge rate than primary battery (non-rechargeable).
Example of primary battery are : lithium metal battery (self life of 10 years) and Alkaline battery (shelf life of 5 years). Example of self discharge rate of a rechargeable battery are : Lithium ion battery (2%-3% per month), Lead Acid battery (4%-6% per month), Nickel Cadmium (15%-20% per month) and nickle metal hydride NiMH (30% per month).
Lithium ion battery have low self discharge is useful when you need to backup and store energy for a longer time duration, however comes with a higher price tag.
Battery Capacity
The capacity of a battery is commonly denotes as Amp-Hour (AH). It basically means that a battery with a capacity of 1 amp-hour should be able to continuously supply a current of 1 amp for 1 hour. In other words supply current of 2 amps for half hour, or 0.5 amps for 2 hours. After delivering the specified amp-hour, the battery will be in a state of fully discharged.
However battery don’t usually work in such linear formula as current and voltage will drop during discharging over time, especially during the near fully discharge state. Hence the amp-hours capacity of a battery is usually specified at a given current in a given time, or assumed to be rated for a time frame period of 8 hours. Similarly to how we calculate energy in kWh.
Battery capacity is different from battery amperage rating. In fact a 100 amp-hours battery doesn’t means the battery can throw out 100 amps current over one hour. It only means that the battery can contain the energy of 100 amp-hours.
Similarly when the power meter tells us we had use 100kWh of energy doesn’t means that we have a 100kW device running over past one hour, as it is a cumulative representation of usage over a period of time that we simplify and express in equivalent usage in one hour.
Depends on battery construction, plate density, internal characteristic and technology, the continuous output of a battery is usually much lower than the amp-hour of a battery. For example if the 100 amp-hours battery have a current specification of 5 amps continuous output, it will output continuous output of maximum 5 amps and will run for nearly 20 hours before the battery become fully discharge.
However the amp-hours of a battery degrades over time due to chemical reaction and build up of internal resistance over time. Hence in actual uses, the battery seems to be become discharge faster after many cycle of use.
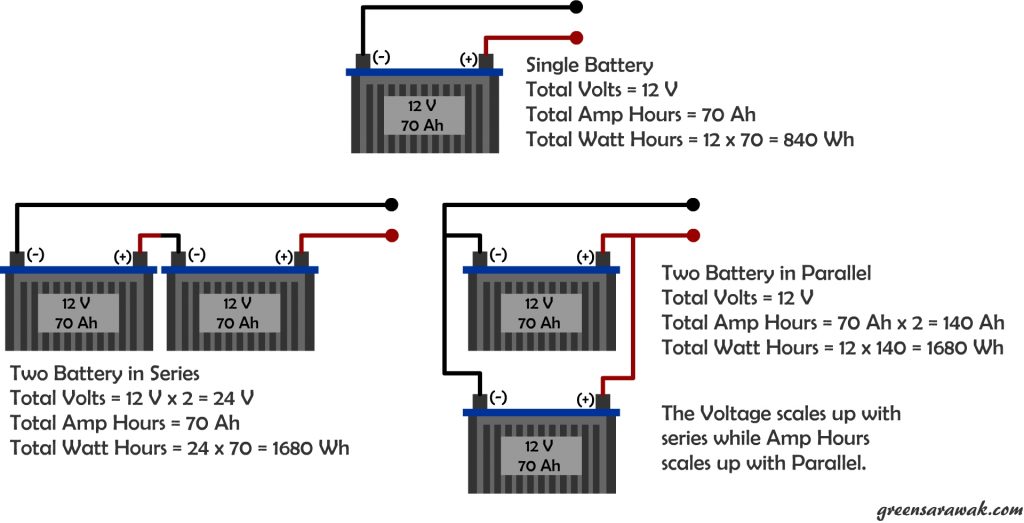
When connecting a battery in a string, the voltage adds up but amp hours doesn’t (just like solar PV panels). Similarly if you parallel them, the voltage remains but Amp Hours increase. In the end the total energy storage will still increase with each adding of battery to the bank as Volts times Amp Hours is Watt Hours.
Charging Temperature and Discharging Temperature
Battery do works in wide range of temperature, but most are manufactured to work well on standard test condition, which is 25 degree Celsius. At extremes of temperature, the efficiency of the battery will be affected, both charging and discharging. It is mainly due to the temperature effect on chemicals inside a battery.
A practical advice for using battery powered electronic devices like digital cameras in sub zero freezing temperature is to take out the battery and warm in palms first before using will allow longer usage. It is the same as solar batteries.
Each different type of battery will have different charging and discharging temperature. Batteries can be discharged over a larger temperature range but charge at a more limited temperature range. For best results, to charge battery between 10 – 30 degree Celsius.
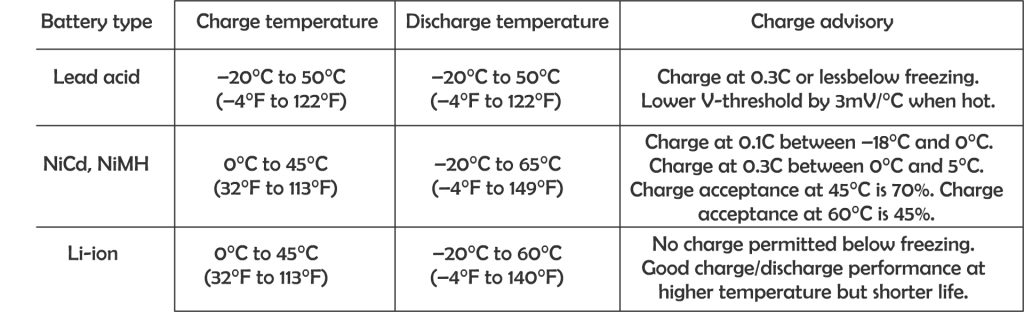
Temperature compensation
Battery charging voltages should be corrected based on battery temperature. This adjustment is referred to as temperature compensation, a charging feature that helps ensure that a battery is neither undercharged nor overcharged regardless of battery temperature.
Temperature affects the internal resistance and charge acceptance. At extreme cold or extreme heat, the charge acceptance reduced, and hence need to be brought to a moderate temperature before charging.
The standard test condition is rated at 25 degree Celsius, that is the temperature where most electronic device tested at, including charge controllers, inverters, solar PV panels and batteries. However in actual use, the ambient temperature is much different from the standard test condition.
When the batteries are cold, higher charge voltage is required in order to push current into the battery plates and electrolyte, however warmer batteries require a lower charge voltage to prevent overcharging causing potential damage to valve regulated lead acid (VRLA) cells and reduce unnecessary gassing if flooded cells are used. Voltage compensation prolongs battery life when operating at temperature extremes.
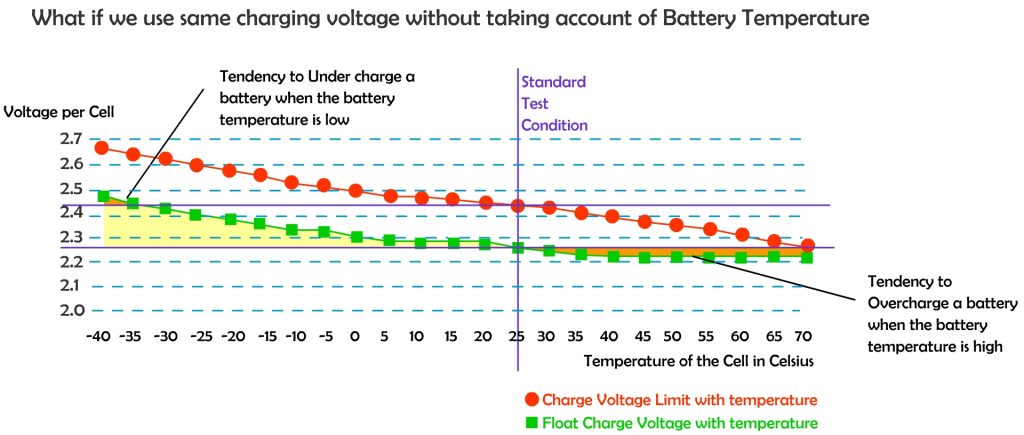
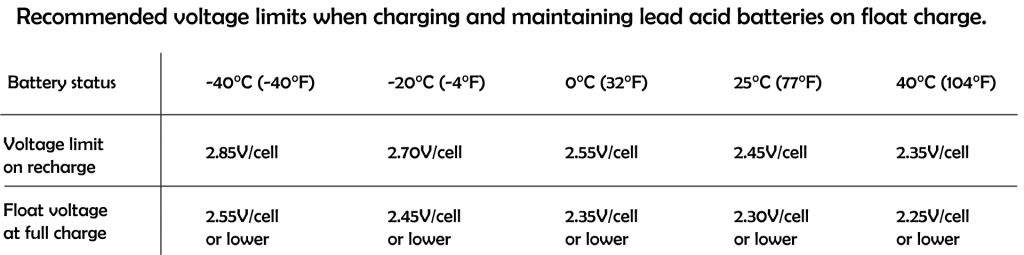
The most widely used temperature compensation formula is: -0.005 V per ºC per 2 V cell. However it still depends on battery type and manufacturer. Different battery types have different characteristic on temperature compensation. It is wise to have a look at the manufacture specification sheet to know the appropriate adjustment required in extreme of temperatures. In fact, some battery manufacturers and charger manufacturers recommend not charging a battery that is 50ºC (122ºF) or hotter.
C-Rates
Charge and discharge rates are often denoted as C or C-rate, which is a measure of the rate at which a battery is charged or discharged relative to its capacity.
A 1 Amp Hours Battery will be charge at C-rate of 1 C if it is charged for one hour with 1 Amps (from empty to full), or discharge at C-rate of 1 C if discharged for one hour with 1 Amps (from full to empty). The same battery discharging at 0.5C should provide 500mA for two hours, or at 2C it delivers 2A for 30 minutes.
Similarly if used in a 10 Amp Hours Battery will be charge at C-rate of 1 C if it is charged for one hour with 10 Amps (from empty to full).
| C-rate | Time |
| 5C | 12 minutes (1/5 hour) |
| 2C | 30 minutes (1/2 hour) |
| 1C | 1 hour |
| 0.5C or C/2 | 2 hours |
| 0.2C or C/5 | 5 hours |
| 0.1C or C/10 | 10 hours |
| 0.05C or C/20 | 20 hours |
| C/x | x hours |
Generally it is advised to charge a battery around 1C or less for safety and longevity reasons. Delivering charge more than 1C can generate heating, gassing, and reduce cell life. However the exact C-rate of the battery can be found in the manufacturing specification sheet of the battery and the charger. Some allow higher C-rate for charging, e.g 2C, 5C, up to 12C with specialized chargers.
When dealing with very high capacity batteries, the C-rate will be much lower due to the fact that it need to handle very high current to have a C-rate of 1 C. (example a 200 Amp Hours Battery will need to handle 200 Amps in one hour if considering 1 C.) As previously known, high current systems will generate allot of wasted heat and is usually less efficient.
Starting Batteries and Deep-Cycle Batteries
Starting batteries is commonly found in automotive to allow electrical ignition (via a electric starters) of a combustion engine. It is also known as an SLI battery (starting-lighting-ignition). These batteries have large amount of thin lead plates with maximum surface area to meet the short instantaneous burst of current required during engine starting. Typically, starting discharges less than three per cent of the battery capacity. After starting of engine, the battery will goes into recharge stage supported by the running engine and the alternator.
Starting Batteries are cheap, but not suitable for solar application, as in solar application there is frequent deep discharges that will cause electrodes to disintegrate and causing premature failure. In solar application there is long float charging during the day but will put the batteries into long partially discharge state throughout the night.
Hence deep cycle batteries is used in solar application, where it have lesser but thicker lead plates that are much less susceptible to degradation due to cycling. They also have higher-density active paste material, thicker separator and plates contain more antimony. Deep cycle batteries are found in electric vehicles (e.g. golf cart), Solar PV systems and Uninterruptible Power Supplies (UPS). These batteries allow a constant current discharges but usually lower peak load current than a starting battery.
Deep-Cycle battery is commonly designed to discharge between 45% to 75% of its capacity, with some able to go as low as 80% discharge. However comparing shallow discharge and deep discharge, the battery will have longer cycle lifespan if Depth of Discharge is low each cycle. For better lifespan vs cost factor, it is advisable to have a average cycle of 45% discharge ( Prevent Depth of Discharge of more than 50%).
Although Flooded Lead Acid Batteries can be of starting batteries or deep cycle batteries type, hence depends on applications, one choose one over the other to ensure your system don’t fails prematurely.
Deep cycle batteries are also available in VRLA, AGM or Gel battery.
The Common type of Solar Battery
The common rechargeable battery for solar battery are
- Standard Lead-Acid Battery or Flooded Battery
- Valve Regulated Lead-Acid Battery (VRLA battery), comes in three type : Sealed Lead-Acid (SLA), Gel Cell, Absorbent Glass Mat (AGM).
- Lithium Ion Battery
Standard Lead-Acid (Flooded) Battery
The standard lead-acid battery is the oldest type of rechargeable battery. It is commonly seen in automobile, and had been one of the most affordable battery with ability to supply high surge current (e.g. to be used to start automobile engine).
Since the battery can be easily been set up to be run in parallel to supply larger amperage or be run in series to supply higher voltage. It is also favorable for large scale off grid backup at lower cost.
In a standard lead-acid battery, it is consist of series of six compartment of 2 volt cells. The 2 volt cell can have voltage range from 1.8 Volt at full discharge (corresponds to ~10.8 Volt) , to 2.1 Volt in an open circuit at full charge (corresponds to ~ 12.6 Volt). The voltage is one of the criteria to decide the state of charge of the battery.
A standard lead-acid battery cell consists of Negative and Positive lead plate sandwiched between an insulator that separates the two different plates to prevent short circuiting. It is flooded and bath in a electrolyte consist of water and sulfuric acid.
Below is a illustration on things happening while discharging or charging a standard lead acid battery.
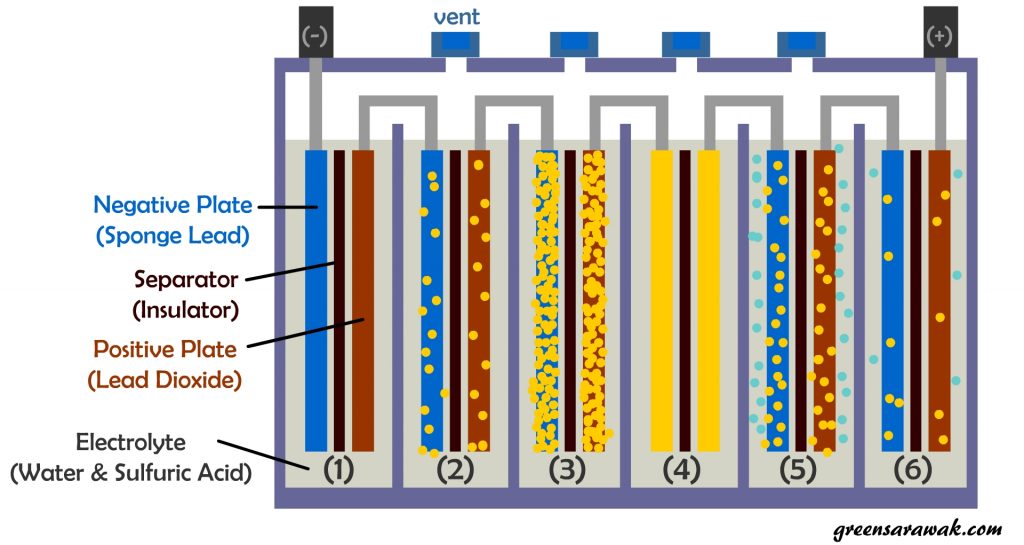
(2) During discharge, chemical reaction between sulfuric acid and the lead plates produces the electricity which also begins to coat both positive and negative plates with lead sulfate (also known as sulfation) .
(3) At fully discharge, the plates is nearly covered with lead sulfate. The battery voltage drop from 12.6 Volt to 10.5 Volt.
(4) Lead sulfate layer is initially soft, it can be reconverted back into lead and sulfuric acid, provided the discharged battery is immediately recharged. If a lead acid battery is not immediately recharged, the lead sulfate will begin to form hard crystals, lead to irreversible loss of battery capacity.
(5) When the battery is recharged, the lead sulfate converted to lead and sulfuric acid. Electrolysis also happens during charging. Water is converted into hydrogen and oxygen (gasses) which need to be vented and causing water loss from battery over time. Hydrogen gas generated during charging is explosive.
(6) Some lead sulfate may still remains on the plates, gradual build-up after each recharging cycle and gradually the battery will begin to loose capacity to store a full charge and eventually must be replaced.
The Pro of Standard Lead-acid Battery:
- Cheap and commercially available.
- Can have variable range of capacity (Some Flooded batteries are available in very high Amp Hours rating).
- Able to dispose large current (peak Load current) when needed (e.g. starting battery for automotive).
- A well maintained flooded cells can last longer than VRLA provided frequently maintain the water level, maintain the charge state and prevent discharge >50%.
- Recyclable. Lead acid batteries are recycled 98% by volume, 99.5% by weight.
The Cons of Standard Lead-acid Battery:
- Sulfaction can significantly reduce the battery ability to store and release energy.
- Unable to sustain a constant current over time due to sulfaction occurs over the plates during discharge, reducing the current flow after some time.
- Depends on specification use of the flooded cells. Automotive battery (starting battery) have large number of thin lead plates that allow instantaneous short discharge of high current burst to meet the sudden peak load during startups, but thin plates are easily damaged by deep discharge causing premature failure. Deep discharges cycles causing thin electrode to disintegrate. Solar applications require deep cycle lead-acid battery which have thicker plates that is much less susceptible to degradation due to cycling.
- Frequent maintenance is needed to make sure the electrolyte is at a good level to flood the cells. Over time the electrolyte will be reduce in volume due to hydrogen and oxygen gasses are released into the air during recharging.
- Have lower discharge-recharge cycle (battery life cycle), worsen if frequently discharge below 50%.
- Higher self discharge rate in compare to other batteries.
- Should always place upright position, else you will risk acid leakage.
- Requiring ventilation around a charging battery. Hydrogen gas released during charging can cause explosion.
- Lead is toxic to the environment and do not dispose to landfills.
Valve Regulated Lead-Acid (VRLA) Battery
The valve regulated lead-acid battery is commonly known as maintenance free battery. It is commercially advertise as battery you can place and forget about it until it finally give way. There is no need to add battery water like flooded cells do. It have similar construction to the Standard flooded lead-acid battery but with slight variation to handle the sulfaction and gassing loss.
The three common type of VRLA battery is :
- Sealed Lead-Acid Battery (Sealed Valve Regulated Wet Cell)
- Absorbent Glass Mat Battery
- Gel Battery
In solar batteries, the commonly used VRLA are AGM or Gel batteries.
Sealed Lead-Acid (SLA) Battery having the same composition as a standard lead-acid battery, except it don’t have a open vent to release the gasses. The battery will retain generated gasses within its battery compartment as long as the pressure remains at a safe level. Under normal operating condition, the gases can be recombine within the battery itself. In some VRLA, gases only released by minimal amounts through a very small vent to regulate pressure back to safe levels when overcharging or when pressure is beyond its safety limits to prevent explosion. In some sense SLA is a Flooded Lead-Acid Battery which is sealed, and doesn’t allow user to add water and hence “maintenance free”.
Absorbent Glass Mat (AGM) Battery (also called starved electrolyte) feature very thin fiberglass mesh between the battery plates which serves to contain and immobilize the electrolyte. The electrolyte is held in the glass mats, as opposed to freely flooding the plates. These very thin glass fibres are woven into a mat to increase surface area to hold sufficient electrolyte (up to 95%) on the cells for use in the cells lifetime. Due to the Glass Mat and Lead electrode is tightly packed, hence have zero plate movement on vibration, which render them immune to vibration. They are also perform well in freezing temperature. However they are more expensive than Gel or SLA counterparts.
Gel cells add silica dust or other other gelling agents to the electrolyte, forming a thick putty-like gel. Commonly found are sulfuric acid mixed with fumed silica. Gelling of the electrolyte reduce electrolyte evaporation, spillage and boast greater resistance to shock and vibration. The antimony in the lead plates is replaced by calcium to allow gas recombination taking place in the cell. (Antimony is alloy with lead in lead to increase the plate hardness and mechanical strength, and increase charging characteristics and reduces generation of unwanted hydrogen during charging). The gas recombination takes place very efficiently in a Gel cells. During overcharging, the oxygen evolved from the positive plate will travel through the gel to negative plate where oxygen gas and hydrogen absorbed on the surface of the sponge lead negative plate are recombined back to water. The valve is regulated at 2 psi, which is enough for a full recombination to take place and prevent gas loss.
Both AGM and Gel cell have their electrolyte immobilize, which is an added benefit for many portable applications in which you can place the battery upright, lying on any direction, and even upside down.
The pros of Valve Regulated Lead Acid Battery:
- Maintenance free ! You don’t need to worry about adding water level to your battery over time (or basically you can’t add anything else).
- Installation in location where regular maintenance can be difficult (e.g. remote area)
- Commonly used for power storage applications such as Uninterrupted Power Supply (UPS) for computer and off grid solar power backup solutions.
- Cycle life is longer than poorly maintained flooded lead acid battery, but shorter than well maintained flooded lead acid battery.
- Able to place on different orientation (especially the AGM and Gel type).
- AGM is Immune to vibration due to tightly packed Glass Mat with Electrode with nearly zero plate movement on vibration.
- AGM can tolerate operation in freezing temperature (suitable for snow mobile).
The Cons of Valve Regulated Lead Acid Batttery:
- Immobilizing agents (AGM, Gel) will impedes the chemical reaction to generate current. This makes them having lower peak power ratings and less useful for application that require brief high-current such as starting an engine (unless stated for starting battery uses).
- The electrolyte cannot be tested by hydrometer to diagnose improper charging.
- More expensive than standard lead-acid battery (AGM, Gel).
- Also requires ventilation but less than a standard lead-acid battery.
- You can’t replenish VRLA cells with water like flooded cells do. Hydrogen gas lost from the VRLA cells can’t easily be replaced, and hence required to over-provisioning quantity of electrolyte. (mainly AGM)
- Heavier than standard lead-acid battery (due to weight from the AGM, Gelling Agents and over-provisioning quantity of electrolyte).
- Voltage regulated charger is needed to maximize the life of VRLA battery (especially AGM and Gel). Over voltage will shorten their life span.
- Discharge significantly less hydrogen gas (which is explosive in nature).
Lithium Batteries
Lithium-ion battery or known as Li-ion battery is a type of rechargeable battery where lithium ions move from the negative electrode to the positive electrode during discharging, and reverse movement from positive electrode to negative electrode during charging.
Lithium-ion batteries are common rechargeable battery found in portable electronics (e.g cell phones, electric vehicles etc.) . It has high energy density with low memory effect and low self-discharge rates.
However pure lithium is highly reactive and can reacts vigorously with water to lithium hydroxide and hydrogen gas. Thus a non-aqueous electrolyte is typically used and sealed from moisture.
Lithium-ions can be combine with different material to have different characteristic depends on applications. Handheld electronics with more concern about weight often use Lithium Cobalt Oxide (LiCoO2), which offers high energy density, but presents safety risks, especially when damaged. On the other hand, if weight is less of concern such as electronic tools or solar applications, Lithium Iron Phosphate (LiFePO4), Lithium Ion Manganese Oxide Battery (LiMn2O4, Li2MnO3, or LMO) and Lithium Nickel Manganese Cobalt Oxide LiNiMnCoO2 or NMC) offer lower energy density, but longer lives and less likelihood of unfortunate events in real world use (e.g. fire, explosion etc.).
Lithium-ion batteries can have safety hazard (fire and explosion) if handle improperly or charge too quickly as they contain flammable electrolyte that may kept pressurized. Hence it need a specialized charger or Battery Management System (BMS) with temperature sensor, overcharge and deep discharge protection. The battery is shut off when the battery voltage is too low, or when extreme of temperatures.
Charging temperature is kept at 0–45 °C for longevity of the cells. Charging is stopped if battery is below freezing temperature as electroplating of metallic lithium can occur at the negative electrode during subfrezzing range which is not removable by repeated cycling. Charging at temperature above 45 °C will degrade battery performance and life. Overheated or overcharged Li-ion batteries may suffer thermal runaway that will lead to leakage, explosion or fire.
The life cycle of Lithium-ion batteries also varies with manufacturer and technology. Most will rated at 500-1000 cycles, with highest up to 10,000 cycles if cell is based on carbon anodes. Degradation is mainly temperature dependent, other than Depth of discharge.
Price of the Lithium-ion batteries had been fallen over the years due to mass production of Electric Vehicles that use Lithium-ion batteries and maturing of manufacturing technology. 18650 size cylindrical cells had been the most popular cylindrical cells that had been use in laptops, home based storage solutions and electric vehicles.
Pros of Lithium-ion batteries
- High energy Density.
- Compact and useful for portable electronics.
- Very Low memory effect.
- Low self discharge rate (1.5-2% per month).
- Longer cycle life than Lead-acid battery.
- Maintenance free.
- Immune to vibration.
- Can placed in any orientation.
- Popular choice for whole house backup solution thanks to brilliantly marketed Tesla Powerwall.
- Can be cheaper alternative in the long run when considering benefits of deeper discharge cycle (DoD of 80%), Longer cycle life (e.g. 1000 cycle at DoD of 80%), less change of batteries if proper handling.
- Using of Battery Management System (BMS) in conjunction with Lithium ion batteries will ensure the safety and longevity of the cells.
- Li-ion batteries contain less of toxic metals than other types of batteries which may contain lead or cadmium. Hence less toxic to the environment.
- The metals (e.g. iron, copper, nickel and cobalt) in a Lithium Ion battery can be recycle.
Cons of Lithium-ion batteries
- Expensive than lead-acid battery.
- Charging requirement is strict in compare to lead-acid battery. Charging temperature is kept at 0–45 °C.
- improper handling, overheating and overcharging may cause thermal runaway, risking explosion and fire.
- When stored for long periods the small current draw of the protection circuitry may drain the battery below its shutoff voltage; normal chargers may then be useless since the BMS may retain a record of this battery (or charger) ‘failure’.
- Lithium-ion cells are susceptible to damage outside the allowed voltage range, even by small voltages (millivolts) can results in premature aging of the cells.
Other Rechargeable Battery Solutions
Rechargeable battery had been evolving over the years due to higher demands on home backup solution and concept to store electricity during cheaper low peak hours rate and release during expensive peak hours rates. Quest for safer, cheaper and yet scale-able system making more and more new solutions surface in the market in near future.
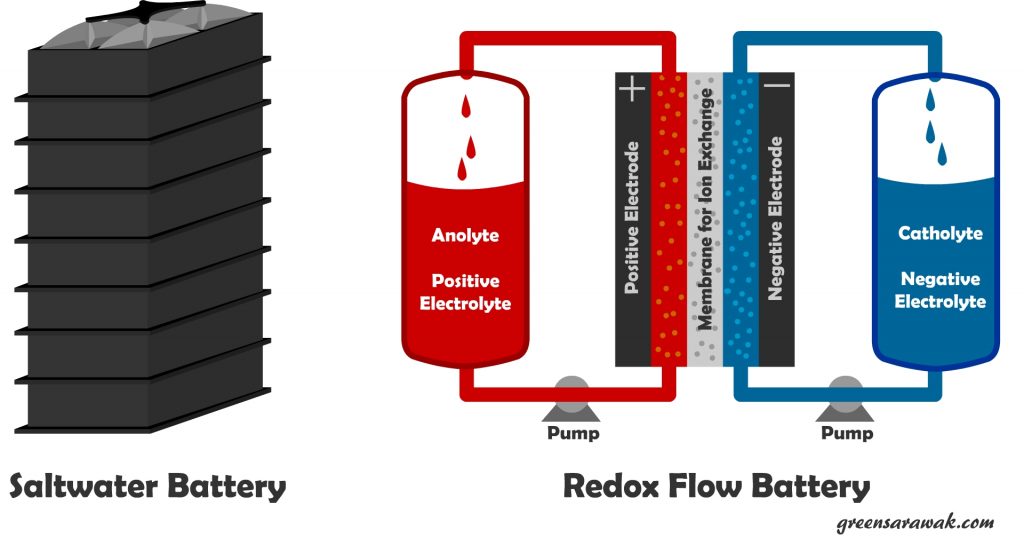
Salt water battery. Yup, a battery that use salt water instead of using heavy metals such as used in lead acid and lithium ion batteries. Saltwater is low cost, non toxic, safe and has no life-reducing side reactions when not in use (low self discharge), allowing longer storage duration. It is popularize by Aquion Energy. In the cell, the high salt concentration allows a solid-electrolyte interphase to form and release more energy with specific energy of 100 Watthour/kg, and can operate with nearly 100% coulombic efficiency at 0.15 C to 4.5 discharge and charge rates.
Since sodium (a type of salt) have high reduction potential, low weight, non-toxic, relative abundance, availability and low cost, it has found its way to many rechargeable battery solutions, Such as Molten-Salt Battery, Sodium Ion Battery, Sodium-sulfer battery etc.
Flow battery or redox (reduction-oxydation) flow battery is a type or electrochemical cell where chemical energy is provided by two chemical components dissolved in liquids contained within the system which is separated by a membrane that allow ion exchange between the two chemicals. These cells are connected to two larger tank (Anolyte Tank and Catholyte Tank) that stores the electrolyte. The stored chemicals are pump through the redox cells where chemical reaction (reduction and oxydation) takes place. Charging a redox cell will reverse the chemical reaction. Such battery is easily scale according to demand but are comparatively less powerful.
Another variant of Flow battery is NanoFlowcell, which have higher energy density with regular redox battery with 600Wh/litre, which allows to be used in automobile.
Flow batteries often come out on top in long-duration storage applications over lithium ion. This is due to their ability to last decades with little maintenance and the fact that the electrolyte materials can be reused or sold.
However all these technology are rather new in compared to age old faithful lead acid battery and well commercialize lithium batteries, it will take some time before more new storage solution take root in home solar backup solution.
Buying a Battery
Before buying a battery and attach to your solar PV system, make sure you already had in mind what battery system you are targeting. There is many things to consider before buying one, else you might regret having the battery system that do not perform as expected, or worst, having injury and fire because of improper handling.
Choose it right – Deep Cycle Battery
Flooded Lead Acid Batteries are cheaper with larger storage capacity than other storage solutions. It is tempting to grab the cheapest one from the store and think it is suitable for solar applications. But wait, are you grabbing a starting battery (for automotive) or Deep Cycle Battery ?
Well, you don’t commonly get deep cycle battery at your local automotive store, unless they also extend their service to electric vehicles such as golf carts. Using starting battery for your solar application will lead to premature failure due to the facts that they don’t support deep discharges and constant current draw.
See the label. If it is a Deep-Cycle Battery, it is suitable for solar applications, as well as other backup solution such as UPS and Whole House Battery Backup.
Make sure your charge controller supports the battery you are buying.
Solar charge controller are a important device in order to maintain and charge the battery. However looking closely into the manufacture datasheet of each charge controller, it will reveal certain important information. Some of the charge controller only accepts Seal Lead Acid, Flooded batteries and Gel batteries, while others extend towards support on Lithium batteries. Some of the advance charge controllers have auto detection function in detection of correct cell type and works accordingly, while others need manual setting.
In Flooded Lead Acid battery settings, the charge controller will have Equalizing charge interval function every month with voltage 14.8V, which is higher voltage than normal charging voltage to dislodge any sulfate crystal on the battery plates. It also allows boost charging around 14.6V and floating charging 13.8V. Flooded batteries have vents that allow gases to be escape from the charging battery, and hence allow more abusive charging voltage.
In Sealed Lead Acid battery, since it is tightly sealed without escaping gases, the Equalizing Charging Voltage is slightly lower at 14.6V. The boost charging voltage is around 14.4V and floating charging voltage at 13.8V. Sealed Lead Acid battery don’t take abusive charging voltage well as it might risk explosion.
In Gel Battery, it doesn’t require equalizing charging voltage. It allows boost charging voltage of 14.2V and floating charging voltage of 13.8V.
In Lithium batteries charging settings, it is the most gentle giant of all. Since there is no acid or gel in the battery, no risk of sulfation, have very long charge retention rate, the Lithium batteries don’t need Equalizing Charging and Float Charging. All it need is a stable boost charging voltage of 14.4V. Remember to buy Lithium battery with its own Battery Management System (BMS) as these gentle batteries are known to last very long and store very high energy density, but improper handling will lead to explosion and early battery failure.
Depends on where you are staying
As we know that temperature affects a battery chemistry, causing it to increase in internal resistance and reducing charging capacity. If you are staying in sub zero Celsius prone area during winter, it is important to get a charge controller that have temperature sensing probe, especially those that can attached to a battery. It will adjust the voltage and charge characteristic depends on battery temperature to prevent undercharging or overcharging.
In winter temperature below zero Celsius, leaving a flooded battery in partially charge state will risk water inside the battery being frozen and causing damage to the battery walls and lead plates (as the more a battery been discharged, the acid will become more watery in characteristic, and frozen water will expand and crack the battery case.)
Lithium battery will stop charging if it is below zero Celsius.
Hence it is crucial to have at least good insulation for the battery when winter months. A good placement will allows a fully functional battery system throughout the winter.
Depends on your system nominals
Voltage nominal is one important factor to consider when comes to battery storage. You can have 6 Volts, 12 Volts, 24 Volts, 48 Volts etc system been set up, but which is the one you need ?
It is first depends on your load. If all your load is 12 Volt rated (e.g LED lights and motors ), it will be easy to go with all 12 Volts system.
But if your load is 12 Volt rated but will drain huge amount of current due to high power (watt) consumption, or requiring higher voltage such as requiring a 12 Volt to 120 Volts or 240 Volts inverter, then it is time to have a battery system that is beyond 12 Volt. Remember that high current draw will result in more heat dissipation and reduce system efficiency.
In short, A 12 Volts system is used for low power consumption application while 24 or 48 Volts is used for higher power consumption application. Once you have set a targeted nominal voltage for your system, you can then buy a charge controller to have the support on the targeted nominal voltage.
What if you have a 48 Volts battery bank but all you have is a 12 Volt LED lamp ? It is easy, just get a step down inverter to convert 48 Volts to 12 Volts and that will do the trick.
Make Sure you know how to maintain your battery
Flooded Battery need to be have frequent check, at least monthly, to make sure it have sufficient acid solution in the battery to prevent damage of battery cell plates due to drying, as these battery release gases during charging, and hence loosing water over time. There are tools to measure the specific gravity of the acid in the battery and you must add only distill water accordingly.
Seal Lead Acid battery and Gel battery usually have less maintenance than flooded battery, but always make sure the physical contacts is good and no oil or dust layers that can reduce the cooling of the cells when charging.
Lithium battery have the least maintenance of all. They can last very long under good operating conditions.
Scaling up your battery capacity
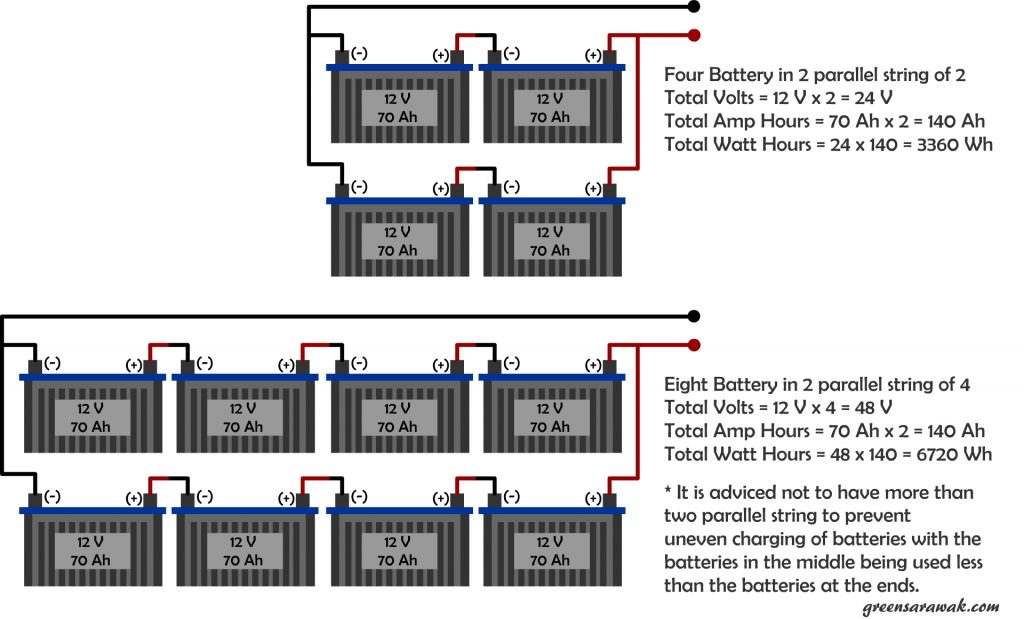
Scaling up your battery can be an easy job by just strapping more batteries together, but it is also more to considered when coming to multiple batteries in your system.
There is not much issues when using one battery, except to worry about how deep you need to discharge the battery which correlates with the cycle life of the battery.
If there is two or more battery, then comes the tricky parts. Each battery have their own internal resistance. Some are higher and some are lower. It is important to add two or more similar batteries together, either in series or parallel. Like wise, don’t mix new and old battery together as internal resistance will change over time due to its chemistry in the battery.
Decide to String or Parallel
In a string configuration, the resistance added up with every addition battery to the string. It won’t cause much a issue with just a single string, but when you decided to have few string in parallel, it is advisable not to overdo with more than two parallel strings.
Even with the same batch, same manufacturer and same battery type, the internal resistance can slightly differ between each other, stringing them together will add up all internal resistance of the battery in that string. Since two set of strings have slightly different resistance, by placing them side by side in parallel will cause more current flow in the string of less resistance (hence work harder), which in turn wear out that particular string faster, and hurting the most hardworking battery first. By having more and more parallel string, more and more resistance comes into play, causing uneven charging and discharging of the battery in the bank.
Likewise, in Lithium battery, although many smaller capacity batteries is placed together in a pack with commonly also in many parallel string, which can be more than two parallel string, especially for large capacity storage solution, It always have dedicate Battery Management System that regulates the charge and discharge of each string, allowing evenly charging and discharging of the battery and hence a longer cycle life. Even with DIY “Tesla Powerwall like” battery bank with many small lithium battery of 18650 type (commonly used in laptop batteries), a careful selection of batteries that have matched internal resistance, self discharge characteristic and voltage characteristic is a must.
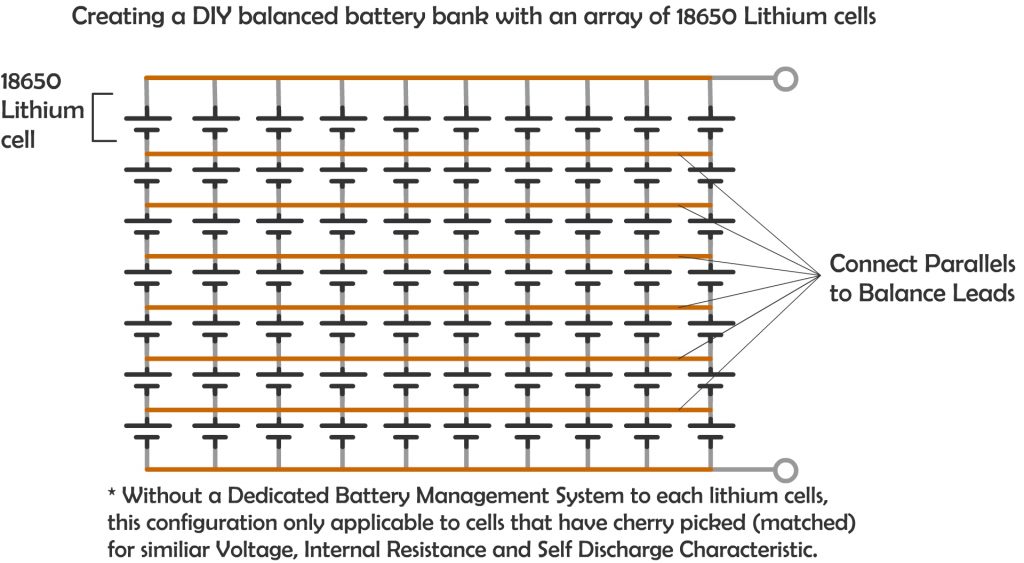
If you really need a higher storage with even more battery to be added to the parallel strings, it is important to even taking consideration of the resistance of the wire that connects the batteries itself. The longer the wire, the higher the resistance.
The concept for balance charging is simple, to ensure the resistance to each parallel battery is as closely as each other. Unless we have tons of battery to test out each battery to find out the internal resistance of each battery, else the next closest thing we can do is to make sure the wire that connect the battery is well balanced.
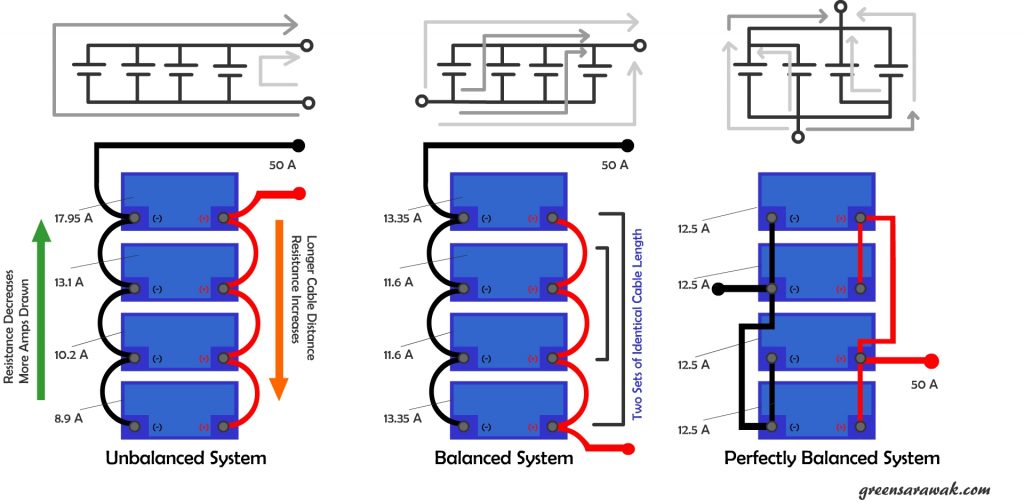
In an unbalanced parallel battery system, there exist multiple path of different wire length connecting each battery, hence there are path with least resistance (short wires) and path with highest resistance (longer wire). This will cause the battery on the path of least resistance to work more and shorten its life span. In a balanced system, the difference in path is minimized and hence to create a near similar cable resistance so that work load is spread more evenly among the batteries.
NEXT CHAPTER >> Know Your Inverters
