Deforestation is an imminent threat to existence of a forest. It was well known that green plants are responsible for changing back the carbon dioxide that we breathe out into oxygen that we breathe in. Will it be the same when all the trees are farmed till the last leaf ? Lets find out.
Oxygen
There is oxygen in the air and that’s why we still survive today.
Back to the basics of science, we had been tought that Oxygen is symbolic by a letter “O” and it have a atomic number of 8. By mass, oxygen is the third-most abundant element in the universe, after hydrogen and helium.
It basically means oxygen is everywhere and near infinate matter of the universe.
Oxygen don’t really stays alone, it is highly oxidizing elements that always stay together with other elements to be stable. As compounds including oxides, the element makes up almost half of the Earth’s crust.
Oxygen cause materials to burn in flames, Oxygen required to run combustion engine in vehicle and generators, Oxygen cause metal to rust, Oxygen reaction is everywhere around us.
At standard temperature and pressure, two atoms of oxygen will bind together to form a much stable dioxygen (O2) or atmospheric oxygen, which constitutes 20.8% of the Earth’s atmosphere.
Three oxygen atoms can forms ozone (O3) , which is abundant at the higher atmospheric level that protect earth surface from high dose of UVB.
With so much oxygen around us, so whats the fuss about oxygen ? We can extract them anywhere. But is it so ? Lets find out how to efficiently extract oxygen from the environment to form the oxygen that we need for breathing and survive.
Evolution of Photosynthesis
Oxygenic Photosynthesis is the main process that produces the freely available oxygen in our earth atmosphere.
Back to the evolution of life on earth, it was speculate that >3500 million years ago the earth atmosphere have no free oxygen. The exact of duration of oxygenic photosynthesis evolved is still not known, but cyanobacteria (bacteria that can obtain their energy through photosynthesis) remain the principal oxygen producer 2500 million years ago. During these time the photosynthesis is not efficient and do not significantly increase the atmospheric oxygen level as O2 produced is mainly absorbed in oceans and seabed rock.
Around 1800 million years ago, Oxygen start to gas out of the oceans but absorb by land surfaces and formation of Ozone layer. Oxygen starts accumulate in the atmosphere since 850 million years ago.
The Great Oxygenation Event suggests that free atmospheric oxygen was first produced by prokaryotic and then later eukaryotic organisms that carried out oxygenic photosynthesis more efficiently.
Oxygen accumulate in the atmosphere, giving raise to opportunity for biological diversification. Aerobic (with oxygen) metabolism is more efficient than anaerobic (without oxygen) pathways. Complex life started nearly 540 millions ago.
Around 300 million years ago (Carboniferous), the atmospheric oxygen is suspected to be around 35%, which is much higher that today’s atmospheric oxygen concentration of 21%.
(Carboniferous means “coal-bearing”, as many coal beds are form during these time. It was found that Carboniferous trees made extensive use of insoluble lignin with very high bark to wood ratio, causing it to be less decomposition. The undegraded carbon built up by these buried dead plants in the soil forms an effective carbon sink, leading to an increase in oxygen levels of atmospheric oxygen. Major climatic events, Carboniferous Rainforest Collapse, glacial ice age, drop in sea level also happens during this Carboniferous period.)
Photosynthesis
Photosynthesis had comes a long way to fill the atmosphere with atmospheric oxygen. Currently the photosynthesis of plants is so efficient that the current atmospheric oxygen concentration can be produced in around 2000 years of photosynthesis.
At current photosynthesis, energy from light is absorbed by proteins called reaction centers inside chloroplasts that contain green chlorophyll pigments. This is what gives leaves their green color.
The photosynthesis process takes in 6 molecules of Carbon dioxide (CO2) and 6 molecules of water (H2O) to form 1 molecule of sugar (C6H12O6) and 6 molecule of atmospheric oxygen (O2).
Photosynthesis is a complex reaction that occurs in two stages – The Light Dependent Reactions and Light Independent Reactions.
Light dependent reactions occur in the thylakoid membrane of the chloroplast. This is the first stage of photosynthesis where light energy splits (photolysis) the Water molecule (H2O) into Oxygen (O2) and Hydrogen Ion (H+) in a photosystems (light dependent protein complex). Subsequent cascade of conversion within lead to the final output of ATP (chemical energy) and NADPH (reducing power).
Light Independent Reaction occur in the stroma of the chloroplast. In plants it is called a Calvin Cycle – a cyclical pathway that involves Carbon fixation, Reduction and Regeneration of Ribulose. The ATP and NADPH generated from the first stage of photosynthesis are required in the second stage of photosynthesis. It is also called the dark reactions as light is not needed. In this stage Carbon dioxide (CO2) is converted into glucose and other products. These products further yield sucrose, starch and cellulose.
Photosynthetic organism converts around 100–115 thousand million metric tonnes of carbon into biomass per year.
Photosynthesis vs Cellular Respiration
Photosynthesis occurs in plants, Algae and Photosynthetic Bacteria. These are organism that can have Anabolic (building up) metabolic process where Carbon Dioxide, Water and Light energy is required to build storage energy such as glucose. It release Oxygen into the air.
Cellular Respiration however happens in all living organism, including humans, animals, bacteria and plants. This is a Catabolic (break down) metabolic process where Glucose and Oxygen is required to form energy currency in the cells (ATP, NADH and FADH2). It release Carbon dioxide into the air.
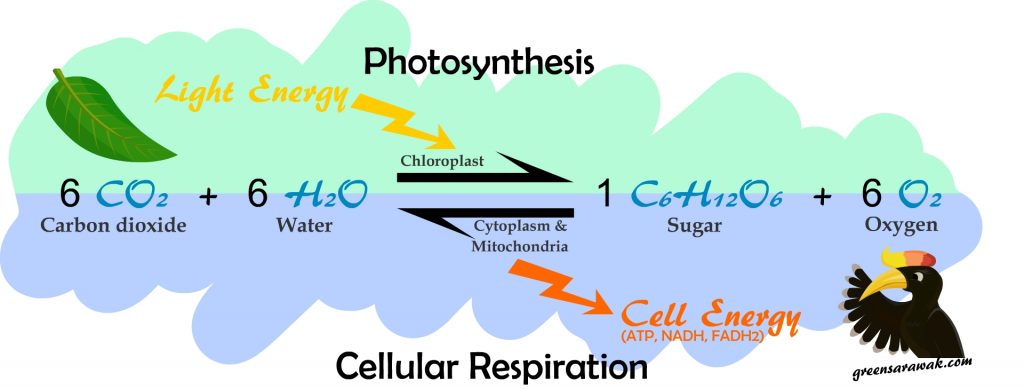
Photosynthesis and cellular respiration are both part of a mutually beneficial relationship. It forms a complete cycle of energy in all carbon based lifeform. Taking one out of the system and both will not survive. Hence it stays in a delicate balance.
The green lungs
Green plants is one of the most efficient photosynthesis organism. They contributed to the atmospheric oxygen concentration around the world.
Green leaved trees are found in most tropical and temperate area. Tropical forests have been called the “Earth’s lungs” or the “green lungs”. Notable Tropical rainforest are Southeast Asia Rainforest (Myanmar, Philippines, Malaysia, Indonesia, Papua New Guinea), Congo Rainforest, Amazon Rainforest, Bosawás Biosphere Reserve, Australia and many of the Pacific Islands.
However these green lungs are receding in size, due to deforestation and expanding of cities and agriculture lands. Frequent forest fire also challenge the greens.
The Water and Oxygen
Water have a chemical formula of H2O. It consist of two hydrogen atoms and one oxygen atom. In liquid form, it is the main constituent of Earth’s streams, lakes, ocean, and runs in body of most living organism. In solid form, it stay in snow, glaciers and ice. In gas form, it can be found in steam and atmospheric water vapor.
Water is one of the resources that most take for granted. Water covers 71% of the Earth surface. Of which, 96.5% of it lies in the seas and ocean, only 2.5% is freshwater. 70% of the freshwater used are going to agriculture.
Water is abundant on earth and easily accessible in most areas. With Oxygen able to dissolved in water (that sustain most aquatic life) and easy to split oxygen and hydrogen apart via electrosynthesis, water has been an alternative to harvest oxygen.
Although oxygen also found in compounds and oxides which makes up almost half of the earth’s crust, harvesting oxygen from these compounds are far more expensive and complex than electrosynthesis.
Photosynthesis VS Electrosynthesis
Both Photosynthesis and Electrosynthesis are a different process, however both of them have the same capability to split (or lysis) a water H2O molecule to Oxygen and Hydrogen. Lets see how it is similar.
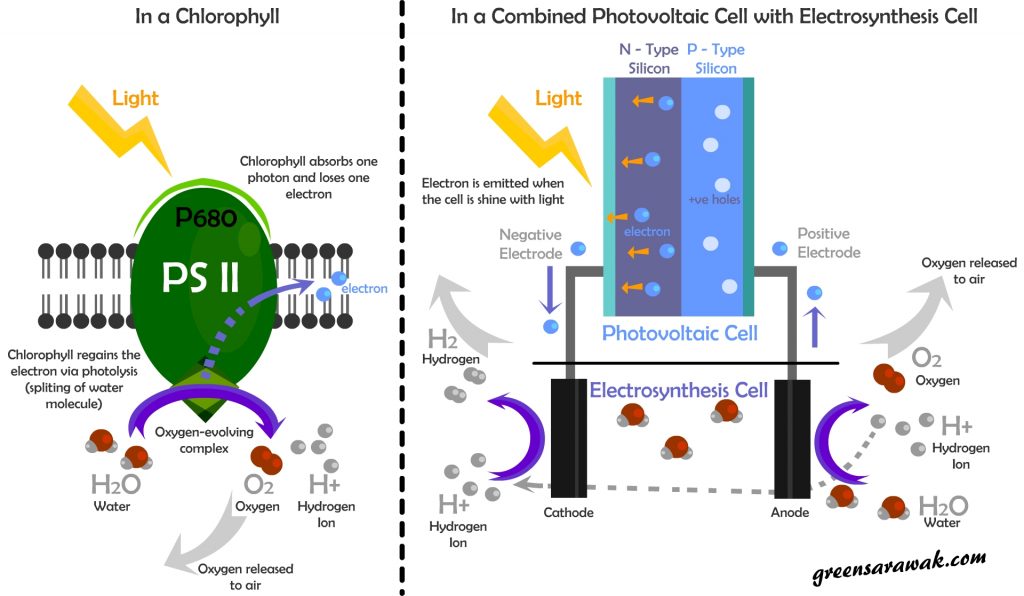
In a first step of photosynthesis, it involves photosystem II, the p680. Once the chlorophyll received one photon , it will liberate one electron to the next chain in their electron transport chain till it finally takes up by NADP to form NADPH. In response losing one electron, photolysis takes place to replace the electron lost. Photolysis will split water molecules into Oxygen and hydrogen ions. Oxygen is released to the atmosphere as waste product while hydrogen ion involves in creating a proton gradient that runs the subsequent steps to generate ATP.
The similar steps can be replicated using a combination of photovoltaic cell and electrosynthesis cell. Photovoltaic cells (usually seen in solar panel that build from semiconductors) have photoelectric properties as when a photon strikes the material with enough energy, it will cause a electric potential by shifting the electrons. The flow of electrons creates current or electricity as we know. When we connect the circuits with a electrosynthesis cell, we can split water to oxygen and hydrogen ions just as photosystem II in chlorophyll do.
In a photosynthesis cell, various combinations and solutions is available, variance including split cell with a membrane/bridge to allow certain ions to move across or a combine cell where solutions are freely mixed. For the purpose of this article we will be quoting the most simplest type of electrosynthesis by just using water (with some salt for increasing conductivity) and two graphite (carbon) electrodes. When the circuit is completed, the side where electron are removed – the anode – will gain back the electron by splitting water to oxygen gas and hydrogen ions. The hydrogen ions is subsequently moves to the cathode where electrons are piling up and will forms hydrogen gas.
In both systems, electron movement is the one that cause the splitting of water to forms oxygen and hydrogen.
As water is much abundant and easy accessible, it will be the next in line to mass produce of atmospheric oxygen in replacement of the loosing trees. Do we need to resorting to artificial oxygen generation in the end ? In such stage we will end up paying for the oxygen we breathe.
The Alternative Explanation on Atmospheric Oxygen
Despite the school of thought that atmospheric oxygen is mainly formed through photosynthesis, however there are other school of thought regarding the formation of atmospheric oxygen. It was argue that despite recent extensive burning of fossil fuels, deforestation, and other extensive human activities, the atmospheric oxygen concentration doesn’t change by much. In biosphere2 experiment shown that the oxygen that are produced by photosynthesis are used up mostly by plants itself and other organism including bacteria, before it can actually replenish the oxygen in the atmosphere.
The most likely explanation is that water molecules that wandered to the outer edges of the atmosphere were breakdown by ultraviolet rays from the Sun. Lighter hydrogen atoms are escaped while heavier oxygen are bound to the earth atmosphere by gravity action, which explains the oxygen accumulation in our atmosphere.
Furthermore, with increase of carbon dioxide in the atmosphere will encourage the exponential growth of plants and increase the biomass formation, which in turn return back the carbon to the biomass in a faster pace and release oxygen in the atmosphere. Over time it will still be balanced out.
Oxygen levels in a global trend
By looking at air trapped inside ancient polar ice sample, Scientist suggest that atmospheric oxygen levels have fallen by 0.7 percent over the past 800,000 years. A 0.7 percent decline in the atmospheric pressure of oxygen may ressemble its concentration at about 100 meters (330 feet) above sea level — that is, about the 30th floor of a tall building.
It may not have drop enough to trigger any major problems for life on earth in the new study. However it may or may not cause by deforestation or burning of fossil fuels as atmospheric carbon dioxide level have not, on average, changed over 800,00 years. Due to atmospheric oxygen level are controlled by complex global systems that tend to regulate and dampen the large swing of its concentration.
Other suggestion are when Ocean cools, the solubility of oxygen increased, storing more oxygen at colder temperature. Alternatively, global increase in erosion rates of pyrite and organic carbon can also led to a steady decline of atmospheric oxygen level. When trapped organic matter becomes exposed on land via deforestation and land erosion, it will react with atmospheric oxygen and lowering the oxygen level in the air.
Oxygen and the climatic effect
climate scientist Chris Poulsen had modify a climate model to test the oxygen and its global climate impact had found that oxygen concentration indeed have impact through series of feedbacks.
“Reducing oxygen levels thins the atmosphere, allowing more sunlight to reach Earth’s surface.”
When the oxygen concentration are higher, the atmosphere gets thicker and scatters more sunlight, and hence less water vapor (which can cause greenhouse effect) been evaporated to trap heat.
However in current day climatic change is not due to oxygen concentration but due to levels of other greenhouse gases such as carbon dioxide and methane are rising dramatically.
Oxygen levels are dropping today indeed are dropping at a very slow rate which approximately tens of parts per million per year, which is much too slow to effect climate change in the world. Unless we give the planet another million years that atmospheric oxygen concentration is of much difference that we need account for oxygen concentration level in the climate model.
Is Lack of oxygen, a myth ?
Although we generally says that the earth having an atmospheric oxygen concentration of 21%, however it is an average out value that do not compare areas of development where usage of oxygen is far from oxygen generation.
Professor Ervin Laszlo says studies shows a dip in atmospheric oxygen level to 19% in impacted areas and it is down to 12-17% over the major cities. These had impact for the human bodily function including organ and immunity system functioning. Long term oxygen deprivation will cause cerebral hypoxia, which leads to a reduced intelligence.
A further dip to 6-7% of atmospheric oxygen level will challenge the sustainability of oxygen dependent organism – humans.
With the lowering of atmospheric oxygen concentration levels in major cities with extensive fossil fuel combustion and lack of trees, it will have further impact on city lives as most city folks stays indoors, which is even more confined space where air is not easily exchanged. Hence, Lack of oxygen is not a myth.
Whats the next step ?
Will our near or far generation encounter a oxygen crisis ? It is a question that is hard to answer. Our atmospheric oxygen concentration is well buffered despite our current expanding human activities. However it is not a reason to harm the environment till the last leaf.
Urban trees and plants do used up precious land in a scenario of major cities as each pieced of wasted land will means millions lost in potential commercial and financial benefit from the land.
Urban trees in most developing cities are also at risk due to expansion of infrastructure, converting tree planting avenues to un-obscure highways for transportation, converting empty lots to parking lots and buildings.
There is a paradigm shift in current building architecture globally. More and more new buildings with incorporated greens are designed and constructed. Some of these comes with roof top gardens with palm trees and shrubs, or plant covering exteriors to reduce the heat.
Other effort by individuals including converting empty lots to public parks with trees will have a long term effects on the sustainability of the environment.
So whats are your green steps ?